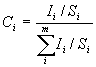X-ray
Photoelectron Spectroscopy (XPS)
[Description] [Instrument] [Sample Requirement] [Application]
X-ray photoelectron spectroscopy, which is also known as electron spectroscopy for chemical
analysis (ESCA), is probably the most widely used surface analysis technique.
This technique is based on the photoelectric effect
and was initially developed by Professor Kai Siegbahn at the University of
Uppsala. When a beam of X-rays is directed to a
sample, the interaction between an X-ray photon and the core level electron
of an atom causes a complete transfer of the photon energy to the electron.
The electron then has enough energy to escape from the surface of the sample.
This electron is referred to as the photoelectron.
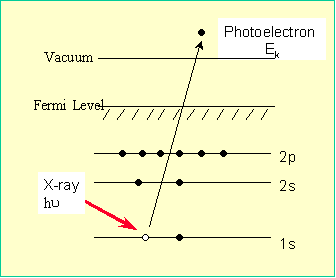
The basic principle of XPS is schematically shown in
Figure 1. For an electrically conductive solid, the binding energy of the
core level electron (
where
where C is a charge constant which is unknown and varies from
sample to sample. Therefore, for insulating materials, the electron binding
energy is usually determined by using an internal reference peak. For
example, the C 1s peak of aliphatic carbon at 285.0 eV is
often used as the internal reference for polymers. Elements have unique electron binding energies. Therefore, knowing the electron binding energy allows the identification of various elements. XPS is thus able to detect all elements except hydrogen. Furthermore, the electron binding energy is also sensitive to the electronic environment of the atom. When an atom is bonded to another atom of an element having a different electronegativity, the electron binding energy may increase or decrease. This change in binding energy is called the chemical shift, which can be used to provide chemical information of a molecule or compound.
Figure 1: Schematic diagram showing the
basic principle of XPS [1] Although
X-rays penetrate deeply into the sample, the photoelectrons can only escape from
a region near the surface. The sampling depth of XPS is given by the
following equation:
where
XPS is also a quantitative technique. The XPS peak intensities after
normalized by the sensitivity factors can be used to calculate the surface
chemical composition by using the following equation:
where Reference [1] C.-M. Chan and L.T. Weng, Reviews
in Chemical Engineering, 16 (2000) 341-408 As XPS experiments are
carried out under ultra-high vacuum, the samples to be analysed must
be vacuum compatible. Both conductive and insulating materials can be
analysed.
|